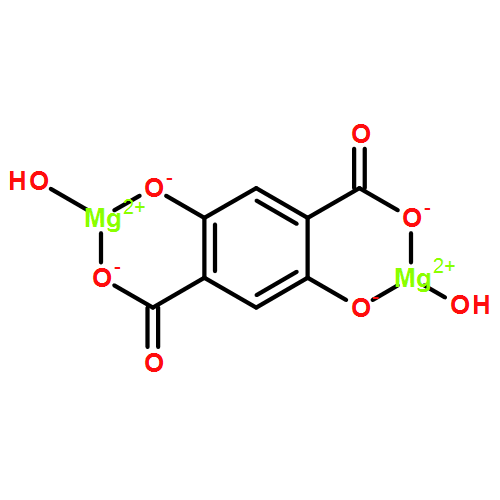
CAS:1565828-96-7
M.F.:C14H16N2O8Mg2
M.W.:388.895
Pricing
//
Product: MOF-74(Mg)
Synonyms: Mg-MOF-74; Mg-DOBDC; CPO-27(Mg); Mg2(dhtp)
CAS:1174268-74-6
Basic Information
| Unit MF. | C8H4O8Mg2 | Unit MW. | 276.72256 | ||
| Coordination Metal | Mg | Linkers | 2,5-Dihydroxyterephthalic acid (CAS: 610-92-4) | ||
| Aperture |
1.2 nm |
Pore volume | 0.65 cm3/g | ||
| Surface Area | BET Specific surface 1100 m2/g, | ||||
| Analog Structure |   |
||||
Product Property
| Appearance | Yellow Powders |  |
||
| Particle Size | 3-5um bulk particles | |||
Stability
1) MOF-74(Mg) is stable in air for weeks, but decomposed gradually in wet and hot conditions.
2) Thermal stability, thermal decomposition temperature above 450 ° C
2) Thermal stability, thermal decomposition temperature above 450 ° C
Preservation
1) Keep sealed in dry and cool condition
2) It is recommended to activate for >6 hours at 150 degree in vacuum.
Other Features
Fluorescence:NA
Applications
1) Gas (e.g. carbon dioxide & CH4) and pollutant air adsorption
2) MOF-74 is instable in acqueous solutions, thus it could be used for drug adsorption and release
2) MOF-74 is instable in acqueous solutions, thus it could be used for drug adsorption and release
Characterizations
References
1) J.K. Nathaniel, L. Rosi, Eddaoudi Mohamed, Banglin Chen, Michael O'Keeffe, Omar M. Yaghi, J. Am. Chem. Soc. 2005, 127, 1504–1518, DOI: 10.1021/ja045123o ; Rod packings and Metal−Organic frameworks constructed from rod-shaped secondary building units
2) Dietzel, Pascal D. C.; Blom, Richard; Fjellvaag, Helmer; European Journal of Inorganic Chemistry 2008, 23, 3624-3632, DOI: 10.1002/ejic.200701284 ; Base-Induced Formation of Two Magnesium Metal-Organic Framework Compounds with a Bifunctional Tetratopic Ligand;
3) Yabing He, Rajamani Krishna, Banglin Chen; Energy & Environmental Science, 2012, 5, 9107-9120; DOI:10.1039/c2ee22858k; Metal-Organic Frameworks with Potential for Energy-Efficient Adsorptive Separation of Light Hydrocarbons;
4)Da-Ae Yang, Hye-Young Cho, Jun Kim, Seung-Tae Yang, Wha-Seung Ahn; Energy & Environmental Science, 2012, 5, 6465-6473; DOI:10.1039/c1ee02234b; CO₂ capture and conversion using Mg-MOF-74 prepared by a sonochemical method;
5)Paul M. Schoenecker, Cantwell G. Carson, Himanshu Jasuja, Christine J. J. Flemming, Krista S. Walton; Industrial & Engineering Chemistry Research, 2012, 51, 6513-6519; DOI:10.1021/ie202325p; Effect of Water Adsorption on Retention of Structure and Surface Area of Metal−Organic Frameworks;
6)Jarad A. Mason, Kenji Sumida, Zoey R. Herm, Rajamani Krishna, Jeffrey R. Long;Energy & Environmental Science, 2011, 4, 3030-3040; DOI:10.1039/c1ee01720a; Evaluating metal–organic frameworks for post-combustion carbon dioxide capture via temperature swing adsorption;
7)Youn-Sang Bae, Chang Yeon Lee, Ki Chul Kim, Omar K. Farha, Peter Nickias, Joseph T. Hupp, SonBinh T. Nguyen, Randall Q. Snurr; Angewandte Chemie International Edition, 2012, 51, 1857-1860; DOI:10.1002/anie.201107534; High Propene/Propane Selectivity in Isostructural Metal–Organic Frameworks with High Densities of Open Metal Sites;


