Home > Product Category > COFs-HOF-POF > COFs by Functions > COFs for Post-Synthesis > Metal-Chelatable COFs
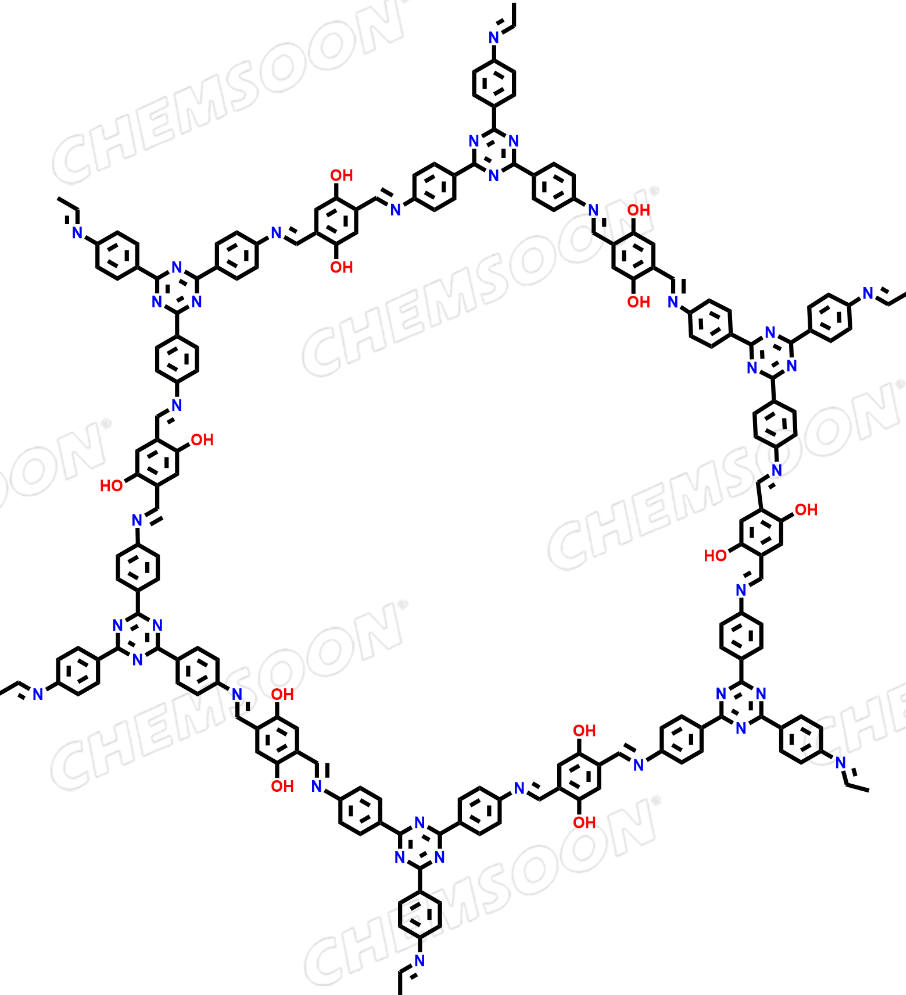
1,4-Benzenedicarboxaldehyde, 2,5-dihydroxy-, polymer with 4,4',4''-(1,3,5-triazine-2,4,6-triyl)tris[benzenamine]
1,4-Benzenedicarboxaldehyde, 2,5-dihydroxy-, polymer with 4,4',4''-(1,3,5-triazine-2,4,6-triyl)tris[benzenamine]
CAS:2102636-16-6
M.F.:C21H18N6.C8H6O4
M.W.:520.53866
Pricing
//
Product: TAPT-DHTA COF
Synonyms:TTA-DHTA COF;TAT-DHBD COF;TzDa COF;1,4-Benzenedicarboxaldehyde, 2,5-dihydroxy-, polymer with 4,4',4''-(1,3,5-triazine-2,4,6-triyl)tris[benzenamine]
CAS:2102636-16-6
Basic Information
| Unit MF. | C21H18N6.C8H6O4 | Unit MW. | 520.53866 | ||
| Linkers |
1)2,5-Diformylbenzene-1,4-diol (CAS: 1951-36-6) 2) (1,3,5-tris-(4-aminophenyl)triazine (CAS:14544-47-9) |
||||
| Aperture | 26.1 和 37.9 Å | Pore volume | 0.44cm³/g | ||
| Surface Area | The BET specific surface area is approximately 600-800 | ||||
| Analog Structure | |||||
Product Property
| Appearance | Orange solid | |||
| Particle Size | / | |||
Stability
1) The thermogravimetric analysis (TGA) of TAT-DHBD under nitrogen atmosphere showed thermal stability up to 250 ° C.
2) After soaking TAT-DHBD in water, acid (1M HCl), and base (9M NaOH) for seven days, its structural integrity was confirmed to remain unchanged by infrared spectroscopy (IR), demonstrating good chemical stability.
Preservation
1) Dry and sealed under normal temperature or low temperature conditions
2) It is recommended to activate for 12 hours at 120 degree in vacuum before gas adsorption test
2) It is recommended to activate for 12 hours at 120 degree in vacuum before gas adsorption test
Other Features
1) Fluorescence:NA;
2) It contains abundant nitrogen sites, including pyridine type and imine nitrogen, as well as phenolic hydroxyl groups, which facilitate the binding of metal ions/metal nanoparticles.
Applications
1) As a catalyst carrier, it is used for carbon carbon bond formation reactions such as Suzuki Miyaura cross coupling.
2) Due to its high specific surface area and porosity, it may have potential applications in gas storage, separation, environmental remediation, membrane separation, and proton conduction.
Characterizations
References
1)Qing Xu, Yanping Tang, Lipeng Zhai, Qiuhong Chen and Donglin Jiang *; Chem. Commun., 2017,53, 11690-11693; DOI: 10.1039/c7cc07002k; Pyrolysis of covalent organic frameworks: a general strategy for template converting conventional skeletons into conducting microporous carbons for high-performance energy storage;
2) Xiaofang Zhao, Junchang Chen, Xuanzhi Mao, Chunyang Li, Linwei He, Fan Zhang, Mingxing Zhang,* Juan Diwu, Guozhong Wu, Zhifang Chai, and Shuao Wang*; Inorg. Chem. 2024, 63, 26, 12333–12341; DOI:10.1021/acs.inorgchem.4c01788; One-Pot Synthesis of a Mixed-Valent Copper(I/II)-Coordinated Covalent Organic Framework Induced by γ-Ray Radiation;
3)Jiamin Zhan, Liangwei Li, Yingjin Ma, Hongming Lou, and Zhixian Li*; ACS Appl. Eng. Mater. 2024; DOI:10.1021/acsaenm.4c00720; Harnessing Copper-Metalated Covalent Organic Frameworks: A Biomimetic Approach to High-Efficiency Dye Degradation



