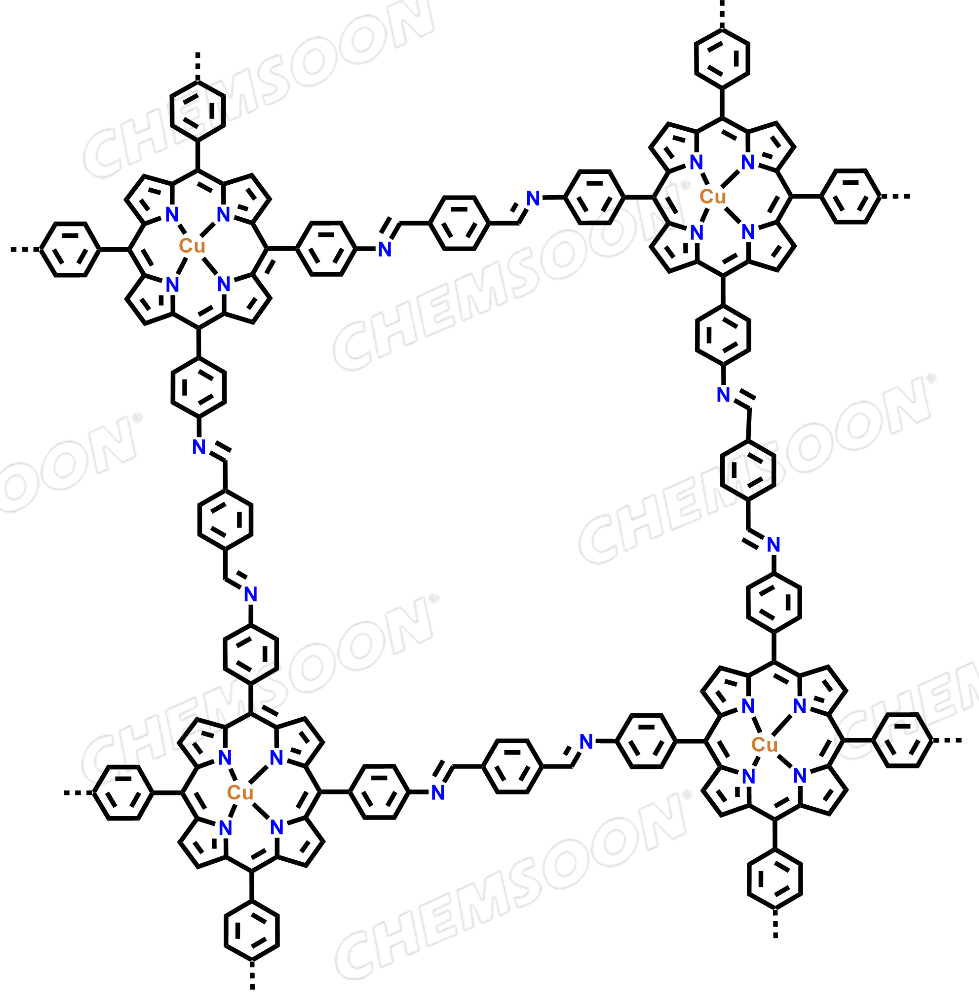
Copper, [[4,4′,4′′,4′′′-(21H,23H-porphine-5,10,15,20-tetrayl-κN21,κN22,κN23,κN24)tetrakis[benzenaminato]](2-)]-, (SP-4-1)-, polymer with 1,4-benzenedicarboxaldehyde
Copper, [[4,4′,4′′,4′′′-(21H,23H-porphine-5,10,15,20-tetrayl-κN21,κN22,κN23,κN24)tetrakis[benzenaminato]](2-)]-, (SP-4-1)-, polymer with 1,4-benzenedicarboxaldehyde
CAS:1417574-84-5
M.F.:C44H32CuN8.C8H6O2
M.W.:870.46
Pricing
//
Product: CuP-Ph-COF
Synonyms:Copper, [[4,4′,4′′,4′′′-(21H,23H-porphine-5,10,15,20-tetrayl-κN21,κN22,κN23,κN24)tetrakis[benzenaminato]](2-)]-, (SP-4-1)-, polymer with 1,4-benzenedicarboxaldehyde
CAS:1417574-84-5
Basic Information
| Unit MF. | C44H32CuN8.C8H6O2 | Unit MW. | 870.46 | ||
| Linkers |
1) TAPP-Cu(2+)(CAS:67595-97-5) 2) Terephthalaldehyde (CAS:623-27-8 ) |
||||
| Aperture | 2.5 nm | Pore volume | 1.11 cm3/g | ||
| Surface Area | BET比表面积约为1185 m²/g | ||||
| Analog Structure |   |
||||
Product Property
| Appearance | brown powder | |||
| Particle Size | 2D layered structure | |||
Stability
1)It does not show significant changes when heated to 300 ° C in air, but under acidic conditions, it may lead to protonation of free radical porphyrins and demetallation of metalloporphyrins.
Preservation
1) Dry and sealed under normal temperature or low temperature conditions ;
2) Suggest using a 60 degree (vacuum) oven for activation for 12 hours before use;
Other Features
Fluorescence:NA
Applications
1) Due to its high crystallinity and porosity, CuP Ph COF may have potential applications in gas adsorption and separation.
2) As an organic semiconductor material, CuP Ph COF may have promising applications in the fields of organic electronic devices and optoelectronics.
3) Its layered structure and adjustable pore characteristics may also make it potentially applicable in fields such as catalysis and energy storage.
Characterizations
References
1) Xiong Chen, Matthew Addicoat, Stephan Irle, Atsushi Nagai, and Donglin Jiang*; J. Am. Chem. Soc. 2013, 135, 546−549; DOI:10.1021/ja3100319; Control of Crystallinity and Porosity of Covalent Organic Frameworks by Managing Interlayer Interactions Based on Self-Complementary π‑Electronic Force;
2) Yu Xia, Guofang Huang, Yan Fan, Xiaolong Zhao, Lei Wang,* Jingwei Huang, Houde She, and Qizhao Wang*; J. Phys. Chem. C 2024;DOI:10.1021/acs.jpcc.4c04463; Preparation of 3D/2D ZnIn2S4/Porphyrin(Cu)-COF Type II Heterojunction: An In-Depth Insight into Interfacial Charge Transfer for Efficient Light-to-Hydrogen Conversion;文章解读