Home > Product Category > COFs-HOF-POF > COFs by Functions > Opteoelectronic COFs > Triazine/BTB-COFs
TAPB-TPA
TAPB-TPA
CAS:1262433-88-4
M.F.:C24H21N3.C8H6O2
M.W.:485.58
Pricing
//
Product: TPB-TP-COF
Synonyms: TAPB-TA COF,SCOF-IC1 ; TAPB-PDA COF;TAPB-TPA COF ;1,4-Benzenedicarboxaldehyde, polymer with 5′-(4-aminophenyl)[1,1′:3′,1′′-terphenyl]-4,4′′-diamine
CAS:1262433-88-4
Basic Information
| Unit MF. | C9H6O3.C6H8N2 | Unit MW. | |||
| Linkers |
1) 1,3,5-Tris(4-aminophenyl)benzene (CAS: 118727-34-7); 2) Terephthalaldehyde (CAS: 623-27-8) |
||||
| Aperture | 3.4 nm | Pore volume | |||
| Surface Area | BET Specific surface >500 m2/g | ||||
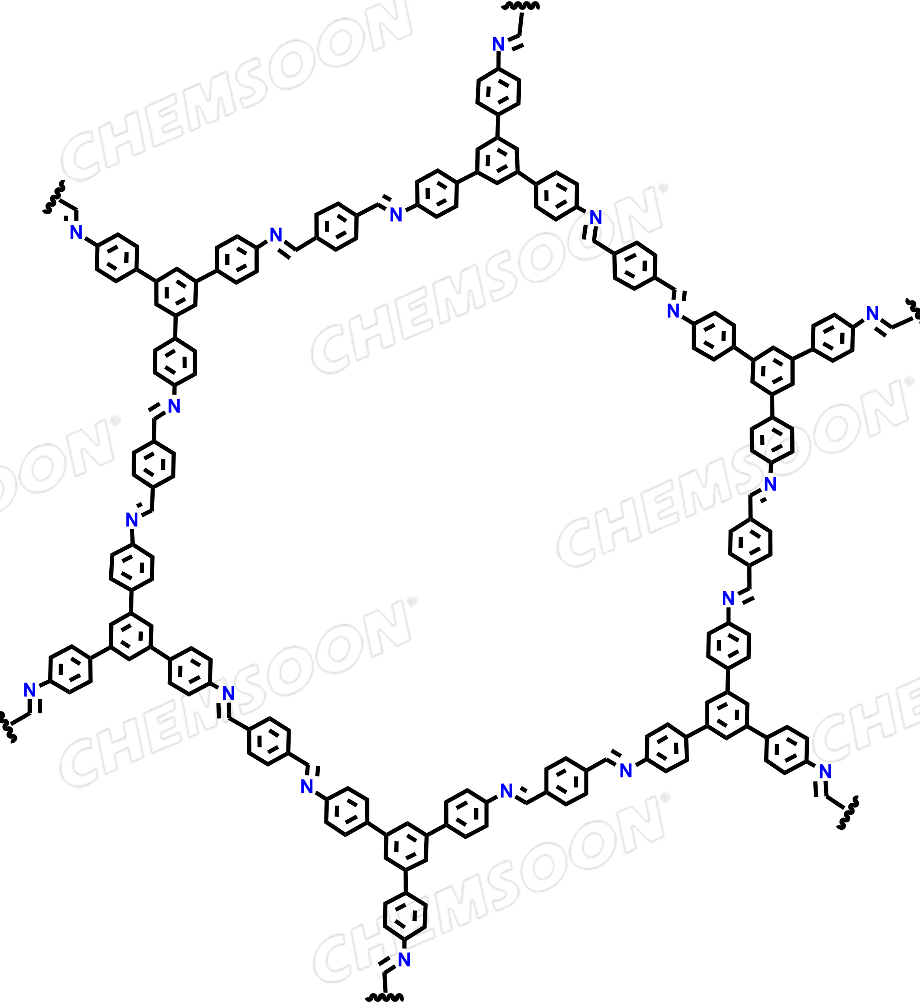
| Analog Structure |  |
||||
Product Property
| Appearance | Orange/Yellow Powders | |||
| Particle Size | irregular cluster particles | |||
Stability
1) TPB-TP COF has good stability, could be stable in air for weeks.
2) Thermally decomposition temperature >350℃
Preservation
1) Keep sealed in dry and cool condition
2) It is recommended to activate for 3 hours at 150 degree in vacuum.
Other Features
Fluorescence:NA
Applications
1) Dry and sealed under normal temperature or low temperature conditions
Characterizations
References
1) X.-H. Liu, C.-Z. Guan, S.-Y. Ding, W. Wang, H.-J. Yan, D. Wang, L.-J. Wan, J. Am. Chem. Soc. 2013, 135, 10470-10474, DOI: 10.1021/ja403464h ; On-Surface Synthesis of Single-Layered Two-Dimensional Covalent Organic Frameworks via Solid–Vapor Interface Reactions
2) P. J. Waller, S. J. Lyle, T. M. O. Popp, C. S. Diercks, J. A. Reimer, O. M. Yaghi, J. Am. Chem. Soc. 2016, 138, 15519-15522, DOI: 10.1021/jacs.6b08377 ; Chemical Conversion of Linkages in Covalent Organic Frameworks
3) H. Xu, J. Gao, D. Jiang, Nat. Chem. 2015, 7, 905-912, DOI: 10.1038/nchem.2352 ; Stable, crystalline, porous, covalent organic frameworks as a platform for chiral organocatalysts
4) B. J. Smith, A. C. Overholts, N. Hwang, W. R. Dichtel, Chem. Commun. 2016, 52, 3690-3693, DOI: 10.1039/C5CC10221A ; Insight into the crystallization of amorphous imine-linked polymer networks to 2D covalent organic frameworks
5) G. Lin, C. Gao, Q. Zheng, Z. Lei, H. Geng, Z. Lin, H. Yang, Z. Cai, Chem. Commun. 2017, 53, 3649-3652, DOI: 10.1039/C7CC00482F ; Room-temperature synthesis of core–shell structured magnetic covalent organic frameworks for efficient enrichment of peptides and simultaneous exclusion of proteins
6) M. Matsumoto, R. R. Dasari, W. Ji, C. H. Feriante, T. C. Parker, S. R. Marder, W. R. Dichtel, J. Am. Chem. Soc. 2017, 139, 4999-5002, DOI: 10.1021/jacs.7b01240 ; Rapid, Low Temperature Formation of Imine-Linked Covalent Organic Frameworks Catalyzed by Metal Triflates;
7)Xuan-He Liu, Cui-Zhong Guan, San-Yuan Ding, Wei Wang, Hui-Juan Yan, Dong Wang*, and Li-Jun Wan*; J. Am. Chem. Soc. 2013, 135, 28, 10470–10474; DOI:10.1021/ja403464h; On-Surface Synthesis of Single-Layered Two-Dimensional Covalent Organic Frameworks via Solid−Vapor Interface Reactions;
8) Cheng Qian,* Anyang Qiu, Qihang Huang, Jiatong Liu, Qiang Gao, Hongwei Wu, and Yaozu Liao*; ACS Appl. Nano Mater. 2024, 7, 8, 9668–9677; DOI:10.1021/acsanm.4c01275; Dynamic Imine Exchange Enables Access to Nanoporous Covalent Organic Frameworks with High Crystallinity for the Heck Reaction;
9) Tianwei Xue, Olga A. Syzgantseva, Li Peng,* Ruiqing Li, Yuyu Guo, Chengbin Liu, Tongxin Qiao, Wenli Hao, Jiaran Li, Lilin Zhu, Shuliang Yang,* Jun Li,* and Wendy L. Queen*; J. Am. Chem. Soc. 2024; DOI:10.1021/jacs.4c10273; Preserving High Porosity of Covalent Organic Frameworks via Functional Polymer Guest Introduction;

